Abstract
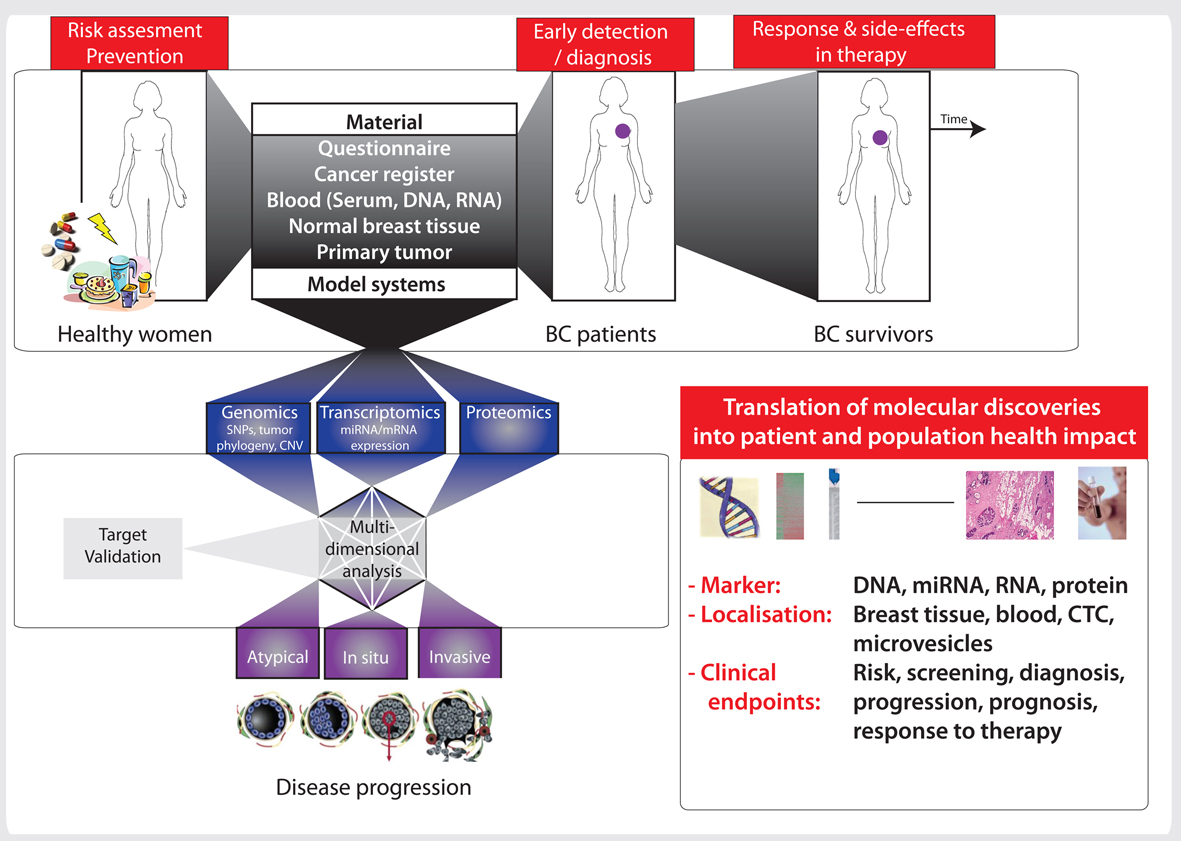
Prospective studies in cancer epidemiology have conserved their study design over the last decades. In this context, current epidemiologic studies investigating gene-environment interactions are based on biobank for the analysis of genetic variation and biomarkers, using notified cancer as outcome. These studies result from the use of high-throughput technologies rather than from the development of novel design strategies. In this article, we propose the globolomic design to run integrated analyses of cancer risk covering the major -omics in blood and tumor tissue. We defined this design as an extension of the existing prospective design by collecting tissue and blood samples at time of diagnosis, including biological material suitable for transcriptome analysis. The globolomic design opens up for several new analytic strategies and, where gene expression profiles could be used to verify mechanistic information from experimental biology, adds a new dimension to causality in epidemiology. This could improve, for example, the interpretation of risk estimates related to single nucleotide polymorphisms in gene-environment studies by changing the criterion of biological plausibility from a subjective discussion of in vitro information to observational data of human in vivo gene expression. This ambitious design should consider the complexity of the multistage carcinogenic process, the latency time, and the changing lifestyle of the cohort members. This design could open the new research discipline of systems epidemiology, defined in this article as a counterpart to systems biology. Systems epidemiology with a focus on gene functions challenges the current concept of biobanking, which focuses mainly on DNA analyses.